« World Intellectual Property Indicators Report: Trademark and Industrial Design Filing Activity Rose in 2019; Patent Applications Marked Rare Decline | Main | Deutsche Bank and Google Cloud Sign Pioneering Cloud and Innovation Partnership »
January 16, 2021
India starts Vaccination with Priority to Healthcare & Frontline Workers

• UPDATE.
• January 16, 2021.
Prime Minister Narendra Modi of India launched the pan India rollout of the COVID-19 vaccination drive via video conferencing. It is the world’s most extensive vaccination program covering the entire length and breadth of the country.

Photo: Health care workers celebrate the 1st phase of the pan India rollout of COVID-19 vaccination drive at Dr. Ram Manohar Lohia Hospital, New Delhi, on January 16, 2021.
• January 09, 2021.
• Oxford Vaccine: India starts Vaccination on January 16, 2021, with Priority to Healthcare & Frontline Workers followed by those above 50 Years and Under-50 with Co-morbidities.
Prime Minister Modi reviews the status of COVID-19 and preparedness for COVID19 vaccination.
The vaccination drive would kick off on January 16, 2021, after the forthcoming festivals, including Lohri, Makar Sankranti, Pongal, and Magh Bihu.
Priority will be given to the healthcare workers and the frontline workers, estimated to be around three crores in number.
They would be followed by those above 50 years of age and the Under-50 population groups with Co-morbidities, numbering around 27 crores.
New Delhi, January 09, 2021 — Prime Minister Modi chaired a high-level meeting to review the status of COVID-19 in the country and the preparedness of the State/UTs for COVID vaccination today.
The Prime Minister took a detailed and comprehensive review of COVID management’s status, covering various issues.
The Prime Minister reviewed the center’s preparedness status in close collaboration with the State and UT Governments for the vaccine’s roll-out soon.
According to the India Ministry of Health and Family Welfare, the vaccination exercise comprises the principles of people’s participation (Jan Bhagidari); utilizing the experience of elections (booth strategy) and Universal Immunization Program (UIP); no compromise of existing healthcare services, especially national programs and primary health care; no compromise on scientific and regulatory norms, other SOPs; and an orderly and smooth implementation driven by technology.
The roll-out of the COVID-19 vaccine will prioritize healthcare and frontline workers. They are estimated to be around three crores, followed by those above 50 years of age and the under-50 population groups with co-morbidities, numbering about 27 crores.
The Prime Minister got apprised about the Co-WIN Vaccine Delivery Management System. The unique digital platform will provide real-time information on vaccine stocks, their storage temperature, and individualized tracking of the COVID-19 vaccine beneficiaries. This platform will assist the program managers across all levels through automated session allocation for pre-registered beneficiaries, their verification, and generating a digital certificate upon completing the vaccine schedule.
The vaccinators and vaccine administrators comprise a crucial pillar of the vaccination exercise. Two thousand three hundred sixty participants were trained during the national level Training of Trainers, including state immunization officers, cold chain officers, IEC officials, and development partners. More than 61,000 program managers, 2 lakh vaccinators, and 3.7 lakh other vaccination team members have trained so far as part of training at States, Districts, and Block levels, the Health Ministry elaborated.
After the detailed review, the Government of India decided that because of the forthcoming festivals, including Lohri, Makar Sankranti, Pongal, and Magh Bihu, the COVID19 vaccination will start on January 16, 2021.
• January 03, 2021.
• People of India receive their New Year Gift; Prime Minister Modi congratulates the Nation as Drugs Controller General of India approves the Oxford Vaccine for emergency use; The University of Oxford welcomes Regulatory Authorisations.


Photo: St. Mary’s College, University of Oxford, Oxford. Image Credit: Billy Wilson.
Oxford / New Delhi, January 03, 2021 — The Drugs Controller General of India today approved the Oxford Vaccine for emergency use. Prime Minister Narendra Modi congratulated the nation and called it a decisive turning point to strengthen a spirited fight against Corona. The approval accelerates the road to a healthier and COVID-free country, he added.
India’s Home Minister Amit Shah hailed the approval of the COVID-19 vaccine granted by the Drugs Controller General of India (DGCI) today. He said the permission to ‘Made in India’ vaccines would prove to be a game-changer. It is a momentous achievement for India, he remarked.
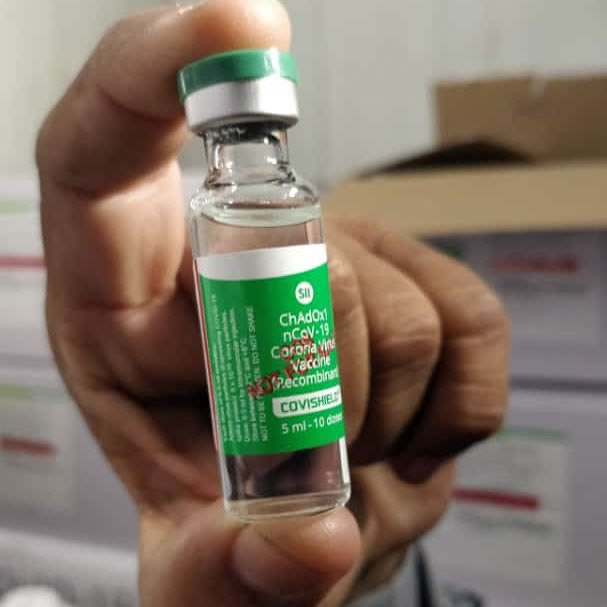
The Serum Institute of India, Pune, is the manufacturing partner of the vaccine candidate named Covishield, developed jointly by the University of Oxford’s Jenner Institute and AstraZeneca.
The Serum Institute of India had selected 17 sites in India to conduct the trial. A total of 1,600 candidates took part in the study.
• The University of Oxford welcomes Regulatory Authorisations:
Meanwhile, the University of Oxford has welcomed the news that the UK Government has accepted the recommendation from the Medicines and Healthcare products Regulatory Agency (MHRA) to authorize the emergency use of the coronavirus vaccine in the UK.
Professor Andrew Pollard, Director of the Oxford Vaccine Group and Chief Investigator of the Oxford Vaccine Trial, said:
‘The regulator’s assessment that this is a safe and effective vaccine is a landmark moment, and an endorsement of the huge effort from a devoted international team of researchers and our dedicated trial participants.’
‘Though this is just the beginning, we will start to get ahead of the pandemic, protect health and economies when the vulnerable are vaccinated everywhere, as many as possible, as soon as possible.’
Professor Sarah Gilbert, Professor of Vaccinology at the University of Oxford, said:
‘This is a day for the team developing the vaccine to celebrate, after a year of tough work under difficult circumstances. The first authorization or use of the vaccine outside of clinical trials has been granted. We still have more to do and will continue to provide more data to multiple regulatory authorities until we can see the vaccine being used to save lives around the world.’
The Oxford vaccine is stable, easily manufactured, transported, and stored at domestic fridge temperature (2-8 degrees C). It can be easily administered in existing healthcare settings, allowing its rapid deployment.
Oxford University’s collaboration with AstraZeneca has been crucial to the successful development of the vaccine and vital for its global manufacturing and distribution across the world. AstraZeneca already has international agreements to supply three billion doses of the vaccine, with access through more than 30 supply agreements and partner networks.
A key element of Oxford’s partnership with AstraZeneca is the joint commitment to provide the vaccine on a not-for-profit basis for the duration of the pandemic across the world and in perpetuity to low- and middle-income countries.
Professor Louise Richardson, Vice-Chancellor at the University of Oxford, said:
‘This is a great day for British science and a great day for universities everywhere. Above all, it is a great day for the many people whose lives will be saved by this vaccine. We are greatly indebted to those who have designed, developed, manufactured, and evaluated it.’
Sources: Prime Minister’s Office, New Delhi. The University of Oxford.
|GlobalGiants.Com|
— The editor holds an Oxford Alumni Card and is a member of the ‘Oxford and Cambridge Society of India.’







Edited & Posted by the Editor | 6:03 AM | Link to this Post







